- About
-
-

- About
-
ACGIH is a 501(c)(3) charitable scientific organization that advances occupational and environmental health.
-
-
- Subscriptions
-
- Science
-
-

- Science
-
This section has been established to help educate industry, government, and the public on what TLVs and BEIs are, and how TLVs and BEIs may best be used.
-
-
- Career Development
-
-

- Career Development
-
ACGIH is committed to providing its members and other occupational and environmental health professionals with the training and education they need to excel in their profession.
-
-
- Publications
-
-

- Publications
-
ACGIH has publications in many different areas that fit your needs in your field.
-
- Publications Store
- ACGIH Signature Publications
ACGIH Digital Library
If you need to purchase the Digital Library, click here.
If you have purchased and need to access the Digital Library, click here.
-
-
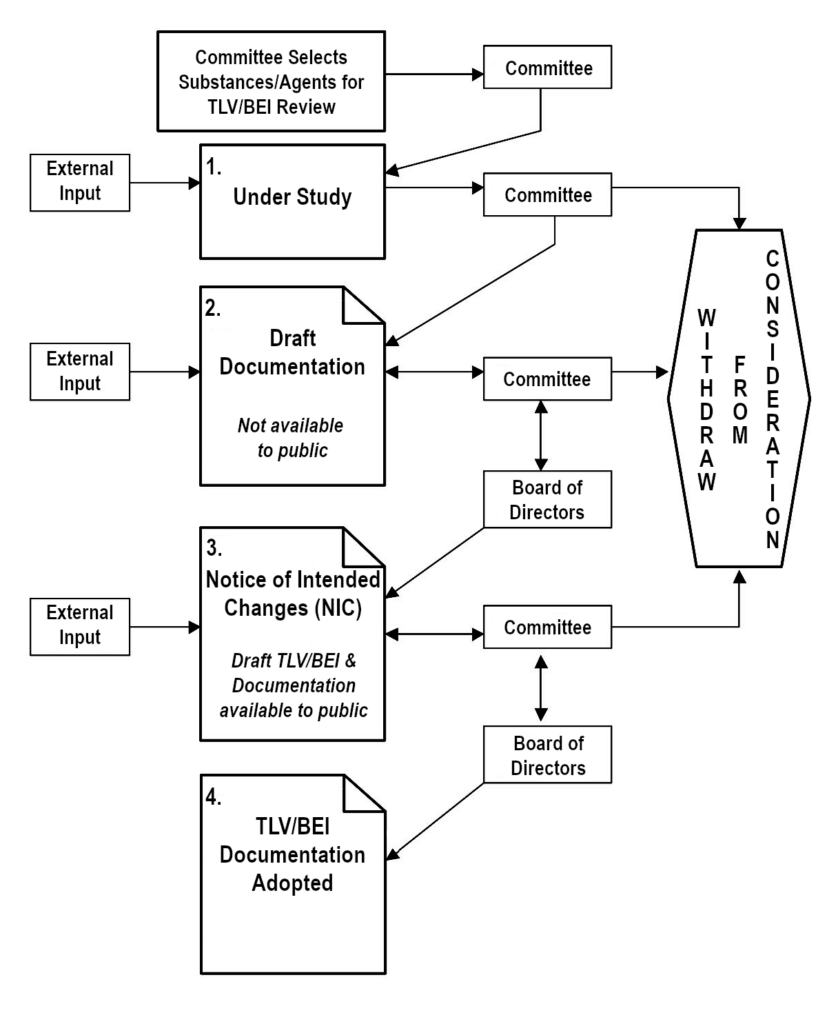
Provided below is an overview of the ACGIH® TLV®/BEI® Development Process. Additional information is available on the ACGIH website (acgih.org). Please also refer to the Process Flowchart at the bottom of this page.
- Under Study: When a substance or agent is being actively researched or written about for TLV or BEI development or revision, the appropriate committee places it on its Under Study list. Each committee determines its own selection of chemical substances or physical agents for its Under Study list. A variety of factors are used in this selection process, including prevalence, use, number of workers exposed, availability of scientific data, existence/absence of a TLV or BEI, age of TLV or BEI, input from the public, etc. The public may offer input to any TLV or BEI Committee by e-mail to science@acgih.org.The Under Study lists serve as notification and invitation to interested parties to submit substantive data and comments to assist the committees in their deliberations. Each committee considers only those comments and data addressing health and exposure issues, not economic or technical feasibility. Comments must be accompanied by copies of substantiating data, preferably in the form of peer-reviewed literature. Should the data be from unpublished studies, ACGIH requires written authorization from the owner of the studies granting ACGIH permission to (1) use, (2) cite within the Documentation, and (3) upon request from a third party, release the information. All three permissions must be stated/covered in the written authorization (see endnote for a sample permission statement). Electronic submission of all information to the ACGIH Science Group at science@acgih.org is preferred and greatly increases the ease and efficiency with which the committee can consider the comments or data.The Under Study list is published on the ACGIH website, updated continuously, and in the TLVs and BEIs book, which is current as of December 1 of the prior year.
- Draft Documentation: One or more members of the appropriate committee collect information and data from the scientific literature, review results of unpublished studies submitted for review, and develop a draft TLV or BEI Documentation. The draft Documentation is a critical evaluation of the scientific literature relevant to recommending a TLV or BEI; however, it is not an exhaustive critical review of all studies but only those pertinent to identifying the critical effect and setting the TLV or BEI. Particular emphasis is given to papers that address minimal or no adverse health effect levels in exposed animals or workers that deal with the reversibility of such effects, or in the case of a BEI, that assess chemical uptake and provide applicable determinant(s) as an index of uptake. Human data, when available, are given special emphasis. This draft Documentation, with its proposed TLV or BEI, is then reviewed and critiqued by additional committee members and eventually by the full committee. This process often results in several revisions to the draft Documentation before the full committee accepts the proposed draft TLV or BEI and draft Documentation. The draft Documentation is not available to the public during this stage of the development process and is not released until it is at the Notice of Intended Changes (NIC) / Notice of Intent to Establish (NIE) stage. Authorship of the Documentation is not disclosed.
- Notice of Intended Changes (NIC) and Notice of Intent to Establish (NIE): When the full committee accepts the draft Documentation and its proposed TLV or BEI, the Documentation and proposed values are then recommended to the ACGIH Board of Directors for ratification as an NIC/ NIE. If ratified, each proposed TLV or BEI is published as an NIC/NIE. At the same time, the draft Documentation is made available through ACGIH Customer Service or online at the ACGIH Publications Store and in the ACGIH DataHub. Following the NIC/NIE ratification by the ACGIH Board of Directors, interested parties, including ACGIH members and other scientific committees, are invited to provide data and substantive comments, preferably in the form of peer-reviewed literature, on the proposed TLVs or BEIs contained in the NIC/NIE. Should the data be from unpublished studies, ACGIH requires written authorization from the owner of the studies granting ACGIH permission to (1) use, (2) cite within the Documentation, and (3) upon request from a third party, release the information. All three permissions must be stated/covered in the written authorization (see endnote for a sample permission statement). The most effective and helpful comments address specific points within the draft Documentation. Changes or updates are made to the draft Documentation as necessary. If the committee finds or receives substantive data that change its scientific opinion regarding TLV or BEI values or notations, the committee may revise the proposal(s) and recommend to the ACGIH Board of Directors that it be retained as an NIC/NIE Documentation.
Important Notice: The comment period for an NIC/NIE draft Documentation and its respective TLV(s), notation(s), or BEI(s) will be limited to a firm 3-month period twice a year, running from January 1 to March 31 and July 1 to September 30. ACGIH has structured the comment period to ensure all comments are received by ACGIH in time for full consideration by the appropriate committee before its spring and fall meetings. Because of the time required to properly review, evaluate, and consider comments during the meetings, any comments received after the deadlines will not be considered in committee deliberations regarding the outcome of the possible adoption of an NIC/NIE. As a general practice, ACGIH reviews all submissions regarding chemical substances and physical agents on the Under Study list, as well as NICs/NIEs, or currently adopted BEI(s) or TLV(s). All comments received before March 31 will be considered in the spring meeting, and those received before September 30 will be considered in the fall meeting. Draft Documentation will be available for review during the comment period. When submitting comments, ACGIH requires that the submission be limited to 10 pages in length, including an executive summary. The submission may include appendices of citable material not included as part of the 10-page limit. It would be very beneficial to structure comments as follows:A. Executive Summary – Provide an executive summary with a limit of 250 words.
B. List of Recommendations/Actions – Identify, in a vertical list, specific recommendations/actions that are being requested.
C. Rationale – Provide specific rationale to justify each recommendation/ action requested.
D. Citable Material – Provide citable material to substantiate the rationale.
The above procedure will help ACGIH to more efficiently and productively review comments.
- TLV/BEI and Adopted Documentation: If the committee neither finds nor receives any substantive data that change its scientific opinion regarding an NIC/NIE TLV or BEI (or notation), the committee may approve its recommendation to the ACGIH Board of Directors for adoption. Once approved by the committee and ratified by the Board, the TLV or BEI is published as adopted in the annual TLVs and BEIs book, and the TLV or BEI Documentation is finalized for print publication and included in the online DataHub.
- Withdraw from Consideration: At any point in the process, the committee may determine not to proceed with developing a TLV or BEI and withdraw it from further consideration. Substances or physical agents withdrawn from consideration may be reconsidered by placement on the Under Study list (step 1 above).
Summary: There are several important points to consider throughout the above process:
- The appropriate method for an interested party to contribute to the TLV and BEI process is through submitting peer-reviewed and public literature. ACGIH strongly encourages interested parties to publish their studies and not rely on unpublished studies as input to the TLV and BEI process. Also, the best time to submit comments to ACGIH is in the early stages of the TLV and BEI Development Process, preferably while the substance or agent is on the Under Study list.
- An additional venue for presenting new data is an ACGIH-sponsored symposium or workshop that provides a platform for public discussion and scientific interpretation. ACGIH encourages input from external parties for suggestions on symposium topics, including suggestions about sponsors, speakers, and format. ACGIH employs several criteria to determine the appropriateness of a symposium. A key criterion is that the symposium must be the most efficient format to present the committee with information that will assist in the scientific judgment used for writing the Documentation and in setting the respective TLVs or BEIs. A symposium topic should be suggested while the substance/ agent is under study, as symposia require considerable time, commitment, and resources to develop. Symposium topic suggestions submitted while a substance is on the NIC/NIE will be considered, but this is usually too late in the decision-making process. A symposium topic will not be favorably considered if its purpose is to provide a forum merely for voicing opinions about existing data. Rather, there must be ongoing research, scientific uncertainty about currently available data, or another scientific reason for the symposium. Symposium topic suggestions should be sent to the ACGIH Science Group (science@acgih.org).
- ACGIH periodically receives requests from external parties to make a presentation to a committee about specific substances or issues. It is strictly by exception that such requests are granted. While there are various reasons for this position, the underlying fact is that the committee focuses on data that have been peer-reviewed and published and not on data presented in a private forum. A committee may grant a request when the data are significantly new, have received peer review, are the best vehicle for receipt of the information, and are essential to the committee’s deliberations. The presentation is not a forum to merely voice opinions about existing data. In order for a committee to evaluate such a request, the external party must submit a request in writing that, at a minimum, addresses the following elements: (a) a detailed description of the presentation; (b) a clear demonstration of why the information is important to the committee’s deliberations; and (c) a clear demonstration of why a meeting is the necessary method of delivery. This request must be sent to the ACGIH Science Group (science@acgih.org). Also, the committee may initiate contact with outside experts (a) to meet with the committee to discuss specific issues or to obtain additional knowledge on the subject and (b) to provide written input or review of Documentation. This contact is only done on an as-needed basis, not as a routine practice.
- ACGIH does not commit to deferring consideration of a new or revised TLV or BEI pending the outcome of proposed or ongoing research.
Important dates to consider throughout each calendar year of the TLV/BEI Development Process:
First Quarter:
Public comments are accepted.^
Committees meet.
Second Quarter:
TLV/BEI Committees vote on proposed TLVs/BEIs for NIC/ NIE or final adoption.*
ACGIH Board of Directors votes on ratification of TLV/BEI Committee recommendations.
Third Quarter:
Public comments are accepted.^
Committees meet.
Fourth Quarter*:
TLV/BEI Committees vote on proposed TLVs/BEIs for NIC or final adoption.
ACGIH Board of Directors votes on ratification of TLV/BEI Committee recommendations.
^ It is recommended that comments be submitted as early as practical, and no later than March 31 or September 30 to allow sufficient time for their proper consideration/review. This is particularly important for an NIC/NIE TLV/BEI.
* These actions typically occur early in the second and fourth quarters but may occur during other times of the year.
Endnote
Sample permission statement granting ACGIH authorization to use, cite, and release unpublished studies:
[Name], [author or sponsor of the study**] grants permission to ACGIH to use and cite the documents listed below, and to fully disclose them to parties outside of ACGIH upon request. Permission to disclose the documents includes permission to make copies as needed.
Example: Joseph D. Doe, PhD, co-author of the study, grants permission to ACGIH to use and cite the document listed below, and to fully disclose this document to parties outside of ACGIH. Permission to disclose the document includes permission to make copies as needed.
“Effects of quartz status on pharmacokinetics of intratracheally instilled cristobalite in rats [unpublished data]. March 21, 2003.”
**This statement must be signed by an individual authorized to give this permission and should include contact information such as title and address.
Last revised June 2023
Enjoy exclusive benefits including free and discounted publications, conferences and continuing education courses – all while supporting the TLVs® and BEIs®.






